The enthalpy of fusion of naphthalene, a crucial thermodynamic property, holds the key to understanding the physical behavior of this aromatic hydrocarbon. By delving into the intricacies of this energy change, we gain insights into the molecular interactions that govern the transition between solid and liquid states.
Experimental techniques, such as calorimetry and differential scanning calorimetry, provide precise measurements of the enthalpy of fusion. These methods reveal the influence of temperature, pressure, and impurities on the energy required for naphthalene to undergo phase transition.
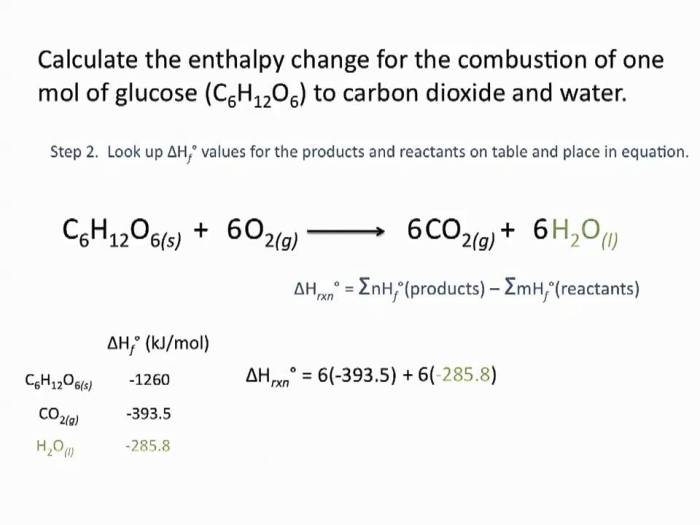
Enthalpy of Fusion of Naphthalene
Enthalpy of fusion refers to the amount of energy required to convert a solid substance into a liquid at a constant temperature. It is a crucial property that provides insights into the intermolecular forces and molecular structure of a substance.
Naphthalene, a polycyclic aromatic hydrocarbon, exhibits a distinctive enthalpy of fusion that plays a significant role in understanding its physical properties and applications.
The enthalpy of fusion of naphthalene is an essential parameter in determining its melting point and other thermodynamic properties. It helps predict the behavior of naphthalene in various processes, such as crystallization, purification, and energy storage.
Measurement Techniques, Enthalpy of fusion of naphthalene
The enthalpy of fusion of naphthalene can be experimentally determined using various techniques. One common method is calorimetry, which involves measuring the heat absorbed or released during the phase transition. In this technique, a known mass of naphthalene is heated in a calorimeter while monitoring the temperature change.
The enthalpy of fusion is calculated from the amount of heat absorbed and the mass of naphthalene.
Another technique is differential scanning calorimetry (DSC), which provides a more detailed analysis of the phase transition. DSC measures the heat flow difference between a sample and a reference material as a function of temperature. The enthalpy of fusion can be determined from the area under the endothermic peak corresponding to the melting process.
Factors Affecting Enthalpy of Fusion
The enthalpy of fusion of naphthalene is influenced by several factors, including temperature, pressure, and impurities.
- Temperature:The enthalpy of fusion decreases with increasing temperature, as the molecular motion increases and intermolecular forces weaken.
- Pressure:Pressure generally has a small effect on the enthalpy of fusion, as the volume change during melting is relatively small.
- Impurities:Impurities can lower the enthalpy of fusion by disrupting the crystal lattice and reducing the intermolecular interactions.
Applications
The enthalpy of fusion data for naphthalene finds applications in various fields:
- Materials science:It helps design and optimize materials with desired melting properties for specific applications.
- Chemical engineering:It aids in process design and optimization for crystallization and purification processes involving naphthalene.
- Energy storage:The enthalpy of fusion of naphthalene is relevant for thermal energy storage systems utilizing phase change materials.
Q&A: Enthalpy Of Fusion Of Naphthalene
What is the significance of enthalpy of fusion in understanding naphthalene’s properties?
Enthalpy of fusion provides insights into the intermolecular forces and energy changes involved in the solid-liquid transition, helping us understand naphthalene’s thermal behavior and physical characteristics.
How does temperature affect the enthalpy of fusion of naphthalene?
As temperature increases, the enthalpy of fusion decreases, indicating a reduction in the energy required for naphthalene to melt. This is due to the weakening of intermolecular interactions with increasing temperature.
What practical applications does the enthalpy of fusion of naphthalene have?
The enthalpy of fusion data is used in designing thermal energy storage systems, optimizing chemical processes, and understanding the behavior of naphthalene-based materials in various applications.