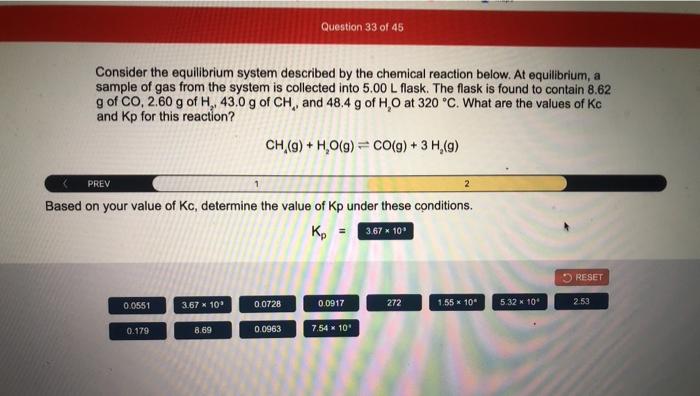
Consider the equilibrium system described by the chemical reaction below. Chemical equilibrium is a fundamental concept in chemistry that describes the state of a system in which the forward and reverse reactions occur at equal rates, resulting in no net change in the concentrations of the reactants and products.
This dynamic equilibrium is characterized by a constant equilibrium constant, which is a measure of the relative amounts of reactants and products at equilibrium.
Understanding equilibrium systems is crucial in various scientific disciplines, including chemistry, biology, and engineering. It enables scientists to predict the behavior of chemical reactions, design experiments, and develop new technologies. In this comprehensive guide, we will delve into the intricacies of equilibrium systems, exploring their thermodynamics, reaction quotients, and practical applications.
1. Equilibrium System Overview
Chemical equilibrium is a state in which the concentrations of reactants and products in a chemical reaction do not change over time. This occurs when the forward and reverse reactions are occurring at the same rate.
The equilibrium constant, K, is a measure of the extent to which a reaction proceeds towards completion. It is the ratio of the concentrations of products to the concentrations of reactants at equilibrium.
Several factors can affect the equilibrium position, including temperature, pressure, and the addition of a catalyst.
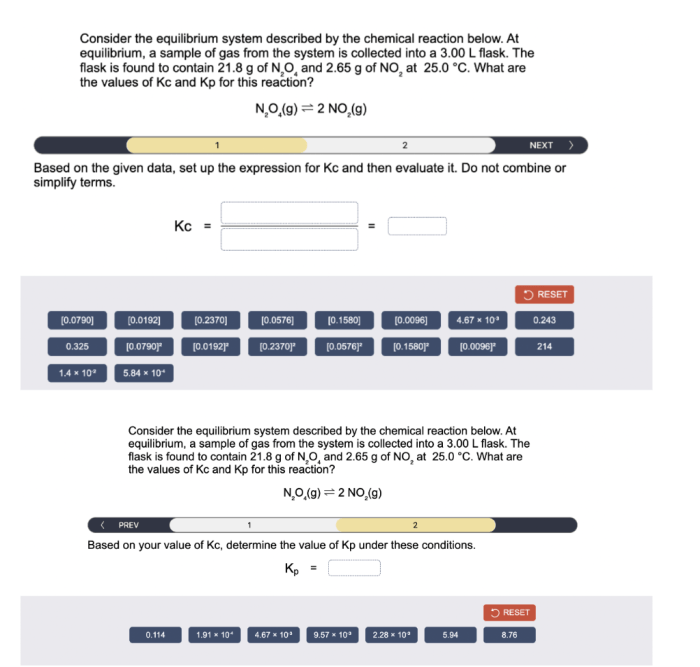
2. Thermodynamics and Equilibrium: Consider The Equilibrium System Described By The Chemical Reaction Below
Thermodynamics plays a crucial role in understanding chemical equilibrium. The Gibbs free energy, G, is a thermodynamic potential that can be used to predict the spontaneity of a reaction.
At equilibrium, the Gibbs free energy change, ΔG, is zero. This means that the system is in a state of minimum energy and maximum entropy.
Changes in temperature and pressure can affect the equilibrium position. Increasing temperature favors endothermic reactions, while increasing pressure favors reactions that produce fewer moles of gas.
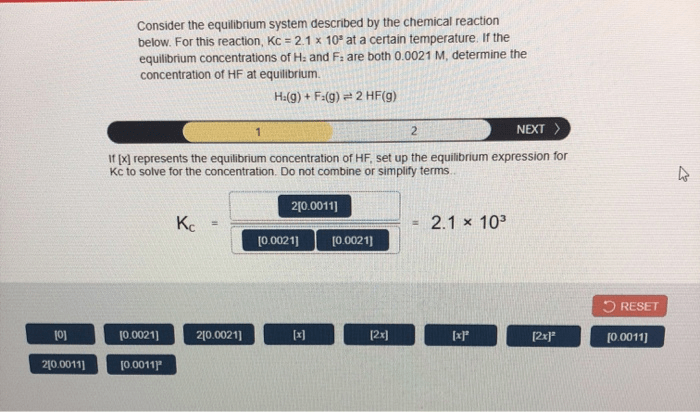
3. Reaction Quotient and Equilibrium
The reaction quotient, Q, is a measure of the relative concentrations of reactants and products at any point in time.
If Q is less than the equilibrium constant, K, the reaction will proceed in the forward direction. If Q is greater than K, the reaction will proceed in the reverse direction.
The reaction quotient can be used to predict the direction of a reaction and to determine whether a reaction will reach equilibrium.
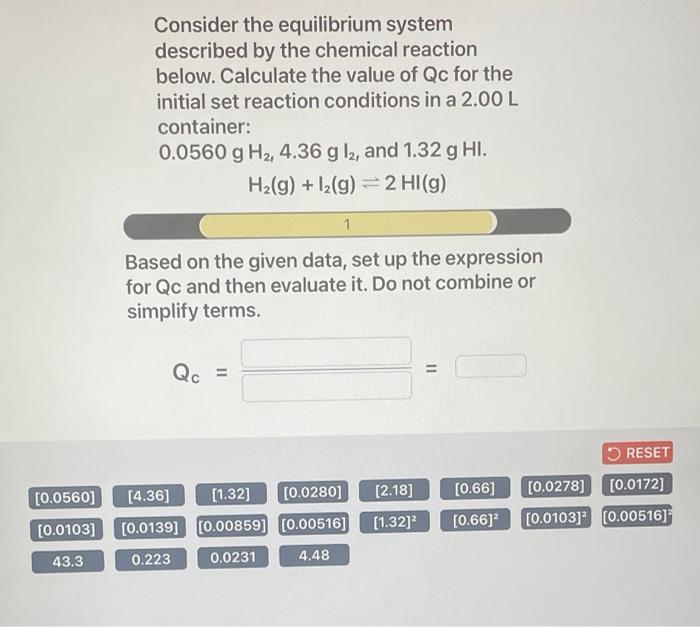
4. Equilibrium Calculations
Equilibrium concentrations can be calculated using the equilibrium constant. The following steps are involved:
- Write the balanced chemical equation for the reaction.
- Set up an ICE table to represent the initial concentrations, changes in concentrations, and equilibrium concentrations.
- Substitute the equilibrium concentrations into the equilibrium constant expression.
- Solve for the equilibrium concentrations.
5. Applications of Equilibrium
Equilibrium is a fundamental concept that has applications in various fields, including:
- Chemistry: Understanding equilibrium is essential for predicting the outcome of chemical reactions and designing chemical processes.
- Biology: Equilibrium plays a role in many biological processes, such as enzyme catalysis and acid-base balance.
- Engineering: Equilibrium is important in designing chemical reactors and other industrial processes.
Answers to Common Questions
What is the equilibrium constant?
The equilibrium constant is a numerical value that represents the relative amounts of reactants and products at equilibrium. It is a measure of the extent to which a reaction proceeds towards completion.
How can I calculate the reaction quotient?
The reaction quotient is calculated using the concentrations of the reactants and products at a given point in time. It provides information about the direction in which the reaction will proceed to reach equilibrium.
What are the factors that can affect the equilibrium position?
The equilibrium position can be affected by changes in temperature, pressure, and the addition or removal of reactants or products.